
Plot Tx1y1 at P=70kPaChoose T between T1sat and T2sat, calculate P1sat and P2sat and use these to calculate x1 by the following eqn. =1 (10.14) If Raoult's Law valid, Pd 1yiPi So lets calculate Pd at z1=0.6 and T=75oC.This is point c in previous Px1y1 diagram. For Raoult's Law, P xiPisat (10.2) P x1P1sat x2P2sat x1P1sat (1 x1)P2satP (P1sat P2sat )x1 P2sat note: a linear line (y=mx+c)Ĭalculate P for a set of x1 and then calculate y1using,ġ83.211750.888074.960.8750.748366.720.6750.569258.470.4750.331350.230.275041.98075y1P(kPa)x1Tī sat liquid solution or bubblepointPb is by BUBL PĬ sat vapor mixtureor dewpointPd is by DEW PĭEW P calculationCalculate Pd and x1, given y1 and T Substitute yi Kixi into (A)zi (1V )xi KixiV xi (1V VKi ) xi (1V (Ki 1))xiĪcetonitrile(1)/Nitromethane(2)Antoine Eqn,Ĭalculate P and y1, given a set of x1 and T=75oCThis is BUBL P calculation. Let T=1 mol, so V and L are mole fractionszi Lxi Vyi zi (1V )xi Vyi (A)
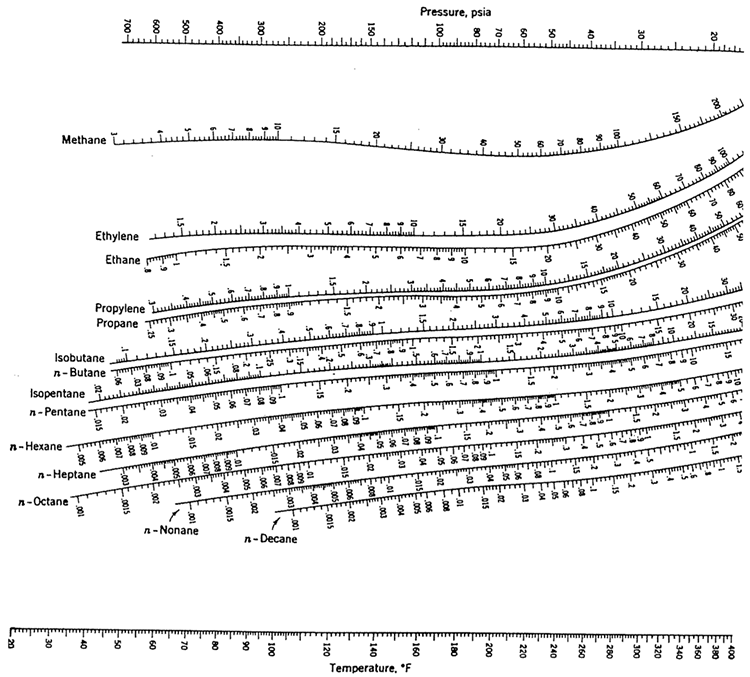
To calculate the T when the last dew disappear as a result of increase in T at constant P. To calculate the T when the 1st dew (drop of liquid) appear as a result of decrease in T at constant P
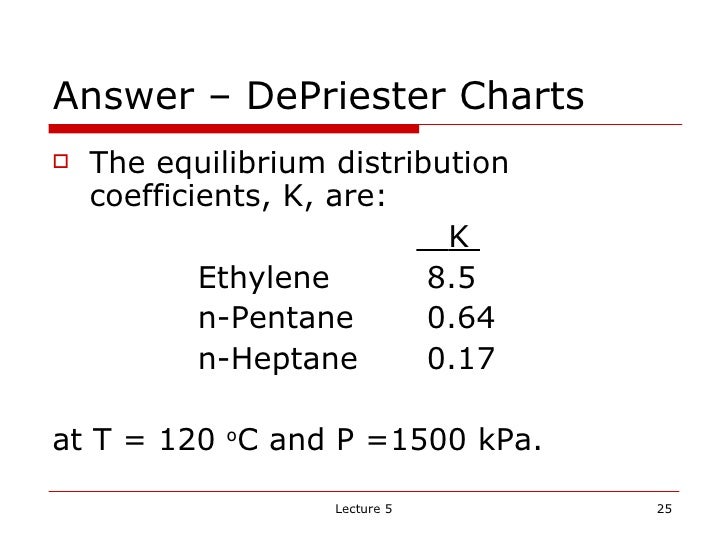
Read value of K-valueat given T and Pe.g.
#How to read a depriester chart how to
It is expected that students have the ability to: Describe the behaviour of VLE and how to simplify the VLE problem. At a pressure of p = 200 psi (1.38 MPa), it would be liquid.SKF 2213 Chemical Engineering Thermodynamics Thus, at a pressure of p = 75 psi (0.52 MPa), pure propane would be gaseous. What is the vapor pressure of propane?Īt a temperature of 60 ☏ (16 ☌), the vapour pressure of propane is 107.71 psi (0.74 MPa). It contracts as temperature declines, and that reduced level of materials in the tank is reflected by the tank level gauge. Propane, like almost all other materials, is affected by cold temperatures. It acts like a float, and will close the valve when it reaches 4.0-4.2 gallons of propane. Legal propane tanks will have an OPD, which is an Overfill Protection Device. It can range from about 60 psi (30 degrees F) to about 200 psi (100 degrees F).

The pressure inside a propane tank is affected by temperature.


 0 kommentar(er)
0 kommentar(er)
